The Department of Inorganic Chemistry guarantees the studies in the fields of Inorganic Chemistry, Bioinorganic Chemistry, and Chemistry for Teaching in bachelor's, master's and doctoral study programmes.
The Department of Inorganic Chemistry guarantees the studies in the fields of Inorganic Chemistry, Bioinorganic Chemistry, and Chemistry for Teaching in bachelor's, master's and doctoral study programmes.
As a part of our research, we focus on the preparation and study of new coordination compounds of transition metals, in particular, on the development of new compounds with medicinal application potential (e.g. substances with antitumour, anti-inflammatory or antidiabetic activity or contrast agents for diagnostic imaging methods) or compounds with interesting magnetic, optical or catalytic properties with industrial application potential (e.g. high-density storage media, various types of sensors).
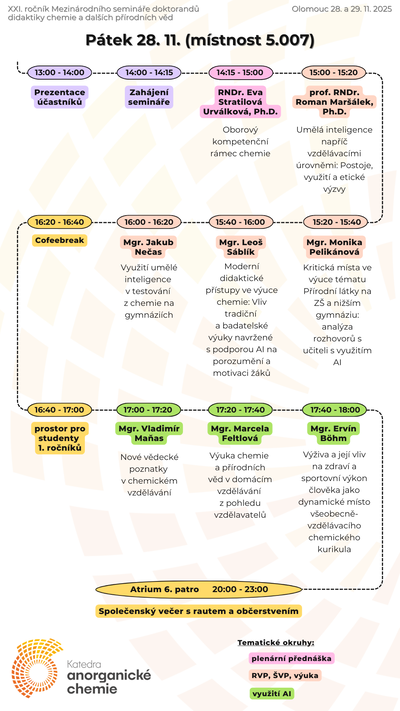
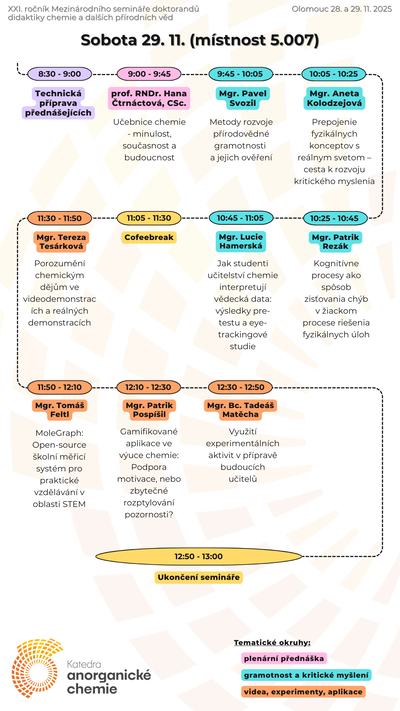
Our teaching activites, as a guarantor of Chemistry for Teaching studies at the Faculty of Science of Palacký University, are also focused on increasing pupils' and students' interest in science and motivation to study science, co-organizing chemical Olympics and also on extracurricular chemical activities for primary and secondary school students.